Log into MRHP through the My Research (MR) portal.
The University of Toronto (U of T) has implemented an automated system for the submission and review of human research ethics protocols—My Research Human Protocols (MRHP)—within the My Research (MR) module. In MRHP, the research protocol flows through a defined review and approval process. It is integrated with the University’s HR system, research funding system, and student information system.
For those who are unfamiliar with human research ethics protocols, or are looking for detailed information on the protocol process or content, please see Ethics in Human Research. To find human ethics guidelines (e.g. informed consent, compensation), please see Human Ethics Principles.
MRHP allows for a more efficient submission process, centralized storage of all documents related to the protocol, and improved capacity to meet tri-agency requirements and other standards.
Faculty and students who are planning to submit a human research ethics protocol are encouraged to log into the MRHP system well in advance of the planned submission date to ensure that they have the required access and that there are no problems with their credentials. For drafting a human ethics application before logging on the MRHP, please use the human participant ethics protocol worksheet.
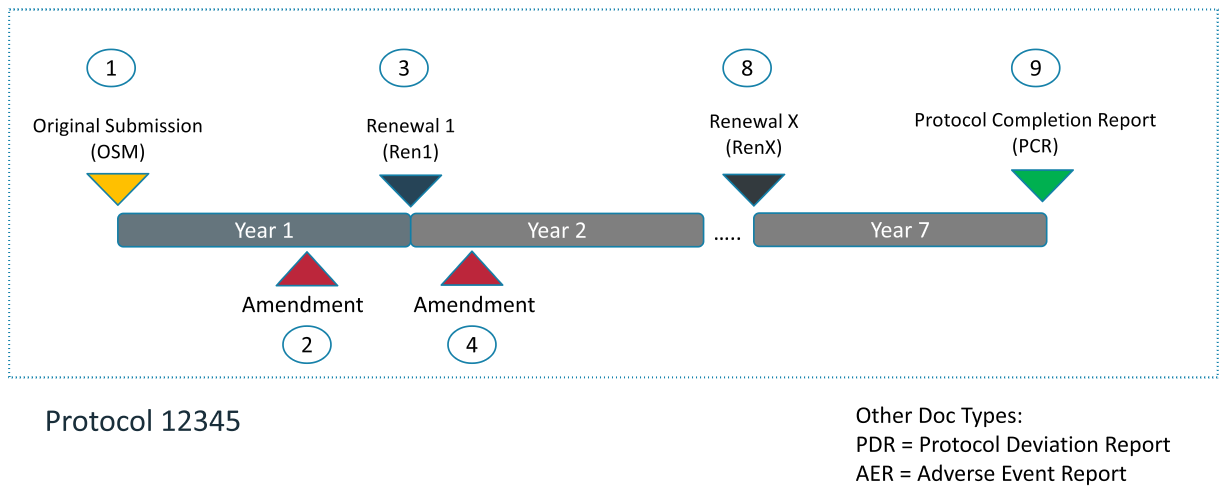
The human research ethics protocol is composed of the following documents.
- Original Submission (first submission)
- Annual Renewal
- Amendment (change to an original submission or renewal)
- Protocol Completion Report (PCR)
The system also allows for the submission of Protocol Deviation Reports (PDRs) and Adverse Events Reports (AERs).
U of T faculty members, students, postdoctoral fellows, and medical residents can be a PI on a human research ethics protocol.
The documents submitted by faculty PIs are sent electronically to the unit head (e.g. Chair or Dean) of the PI’s primary appointment for approval, and are then forwarded to the Human Research Ethics office for assignment to REB reviewers. If the reviewers request revisions, the protocol is returned to the PI for revision and resubmission within the MRHP system.
Documents submitted by student PIs are first sent to their supervisor for approval, then to the unit head of the supervisor’s primary appointment. Finally, they are forwarded to the Human Research Ethics office for assignment to REB reviewers. If the reviewers request revisions, the protocol is returned to the student PI for revision and resubmission within the MRHP system.
Automated email notifications are sent to each person who needs to act on the protocol. The PI is notified when the protocol is returned to them for revision, and when the protocol is approved.
Faculty PIs can submit the following three types of protocols.
- A U of T Investigator protocol for
delegated or full board review - A protocol approved by a Toronto Academic Health Science Network (TAHSN) institution’s ethics board (for administrative review)
- A Course Template (covers all students in a course)
Student/Postdoctoral Fellow/Medical Resident PIs can submit the following two types of protocols.
- A U of T Investigator ethics protocol for delegated or full board review
- A course-based protocol (tied to an individual course)
Within MRHP, faculty PIs can designate a PI Assistant (PIA) to aid in the completion of the protocol. A PIA can be a U of T staff member or an active U of T Student/Post Doctoral Fellow, and they can execute all MRHP-related PI tasks with the exception of document creation and submission, both of which need to be executed by the PI.
Chairs, Directors, Principals and Deans (i.e. those conducting the unit head reviews) can designate an alternate approver for their unit to approve the protocols on their behalf.
| Role | How Assigned | Comments |
|---|---|---|
| Faculty Principal Investigator (PI), Supervisor, Lecturer |
The complete set of faculty roles (including PI, Supervisor, and Lecturer) is assigned automatically to faculty who meet U of T’s PI eligibility requirements, based on their Human Resources (HR) record.
Faculty with Status Only or Clinical Appointments are assigned their roles upon request (please send a request by email to raise@utoronto.ca, and include your U of T personnel number or U of T email address).
External applicants are assigned access manually upon request from the Human Research Ethics Program office to the RAISE system administrator. |
|
| Student PI | Designated by the Supervisor from within the MRHP system (see tips sheets below for step by step instructions) | Must be active U of T student, post doc or medical resident. |
| PI Assistant (PIA) | Designated by PI from within the MRHP system (see tips sheets below for step by step instructions) | Can be admin staff or student. Only faculty PIs can designate a PIA. |
| Unit Head (Chair/Director/Principal/Dean) | Assigned automatically based on HR appointments. | |
| Unit Head (Chair/Director/Principal/Dean) Alternate Approver |
Designated by: Chair/Director/Principal/Dean within My Research Applications (MRA), or by the RAISE team upon request by the Unit Head via email at raise@utoronto.ca.
The alternate approver role is designated only once in MRA and applies to BOTH MRA and MRAP. |
Must be a U of T faculty member |
| Research Ethics Board (REB) member | Assigned by the Human Research Ethics Program office or RAISE system administrator | External members will be provided with a UTORid |
| Other Roles | Assigned by RAISE system administrator |
You can log into MRHP through the My Research (MR) portal.
For Faculty PIs
- Human participant ethics protocol worksheet (for drafting MRHP applications)
- How do I create a new submission (original protocol document)?
- A protocol has been returned to me by the REB for revisions - how do I revise and resubmit?
- How do I create an amendment?
- How do I create a renewal?
- How do I create a protocol completion report?
- How do I designate a PI assistant? (They will help develop my protocol)
- How do I let my Co-applicant(s) edit/develop my protocol?
For Supervisors
- How do I designate a student PI? (They will create their own protocols)
- How do I review my student PI’s protocol?
- How do I extend a Student PI assignment?
For Students
Help Desk
Please contact the RAISE Helpdesk for system support or with questions about how to get access to the system.
- 416-946-5000 (Monday to Friday, 9AM to 5PM)
- raise@utoronto.ca
For Faculty PIs
- Human participant ethics protocol worksheet (for drafting MRHP applications)
- How do I create a new submission (original protocol document)?
- A protocol has been returned to me by the REB for revisions - how do I revise and resubmit?
- How do I create an amendment?
- How do I create a renewal?
- How do I create a protocol completion report?
- How do I designate a PI assistant? (They will help develop my protocol)
- How do I let my Co-applicant(s) edit/develop my protocol?
- Provide and facilitate system access
- Provide training and user clinics
- Create tip sheets and other user documentation
- Provide user support via the RAISE Helpdesk
- Perform system administration
- Work with EASI team on development of system and issue resolution





