BACKGROUND
Serological testing is important for understanding the spread of the SARS-CoV-2 coronavirus by determining how many people in a given population have been infected. The tests are also used to evaluate the responses to current vaccines against this new coronavirus in people who have already been vaccinated. Though several serological tests have been proposed or are in development, the gold standard is still the enzyme-linked immunosorbent assay (ELISA). However, this test is time-consuming (~ 6 hrs), tedious (requires multiple washes), and relatively expensive (~$25/test) offering an opportunity to emerging serological tests of comparable quality for probing immune response.
TECHNOLOGY
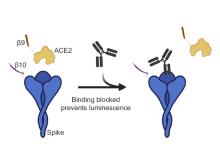
Researchers from the University of Toronto have developed a Serological Assay based on split Tripart Nanoluciferase to detect the activity of neutralizing antibodies against SARS-CoV-2, called neu-SATiN, with potential uses towards COVID-19 disease and vaccine management. This serological assay gives a readout when three nanoluciferase fragments combine to achieve luminescence (Figure 1). Two of the fragments (β9 and β10) are attached to the ACE2 receptor and the SARS-CoV-2 spike protein. Addition of the third fragment reconstitutes the nanoluciferase protein in the absence of neutralizing antibodies; the converse occurs in their presence resulting in a proportional loss in signal.
Figure 1: Neu-SATiN assay for detecting anti-SARS-CoV-2 neutralizing antibodies. Interaction between the ACE2 receptor and the Spike protein results in assembly of the nanoluciferase protein in a proximity dependent manner. Presence of neutralizing antibodies prevents this interaction resulting in a reduction in signal.
COMPETITIVE ADVANTAGE
- Identifies neutralizing ability of anti-SARS-CoV-2 antibodies.
- Directly performed on human serum (no washes needed)
- Rapid (6x faster), low cost (10x cheaper), and quantitative detection
- Very small serum volume needed (~ 5µl)
- Modular assay – easily adapted to new variants
APPLICATIONS
- COVID-19 disease and vaccine management
- Serosurveillance
INTELLECTUAL PROPERTY STATUS
- PCT Application (June 2022)
PROJECT STATUS
Proof-of-concept studies demonstrate the ability to identify the neutralizing activity of SARS-CoV antibodies in human serum samples. Results were benchmarked to commercial neutralization tests. Use of Neu-SATiN for drug screening and serosurveillance demonstrated.