BACKGROUND
Balloon angioplasty, usually accompanied with a stent, is used to widen narrowed or obstructed blood vessels in peripheral and coronary arteries. However, restenosis, the re-narrowing of blood vessels, often occurs in patients following the intervention, due to vascular remodelling caused by the proliferation and migration of smooth muscle cells. Further, there are many peripheral applications where stents are not clinically practical. Currently, this pathological remodeling event can be mitigated by introducing the gradual release of either of two classes of drugs (Taxane and Limus) from drug-eluting stents (DESs) and balloons (DEBs). However, these drugs have general cytotoxicity leading to cell death, endothelial dysfunction and late thrombosis.
TECHNOLOGY
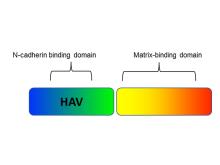
Researchers at the University of Toronto have developed a non-toxic chimeric peptide (Figure 1) to prevent restenosis following angioplasty. The peptide is composed of one domain that attaches to N-cadherin adhesion molecules on smooth muscles cells (SMC), and a second domain that anchors the peptide to the extracellular matrix. The agent prevents SMC migration, without compromising endothelial cell function and regeneration, therefore avoiding the downstream problems of currently used drugs. Additionally, our researchers have developed a nanoparticle polymeric drug delivery system which enables the release of the chimeric peptide from angioplasty devices (stents and balloons). The nanoparticles enable high peptide loading under gentle conditions (i.e. no denaturation) and form a film that can be readily deposited on drug-eluting devices (stent, balloons, catheters), and enable delayed and controlled release of the peptide from the nanoparticle once delivered to the tissue via the devices.
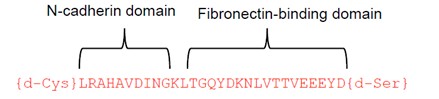
Figure 1. Chimeric peptide schematic. It is composed of an N-cadherin domain to bind to smooth muscle cells and a matrix-binding domain that attaches to the extracellular matrix.
COMPETITIVE ADVANTAGE
- Chimeric synthetic peptide for prevention of restenosis
- Efficacious
- Non-toxic - Vascular cells treated in vitro with peptide are viable.
- Excellent cell selectivity – inhibits smooth muscle cell migration by at least 60%, does not inhibit endothelial cell migration using in vitro assays.
- Inhibits intimal thickening after balloon angioplasty, but does not compromise endothelial healing or function
- Amino-acid and degradable polymer base that naturally resorbs with minimal foreign body response
- Companion drug delivery system developed
- High peptide loading
- Gentle on biologics (i.e. no degeneration)
- Ideal drug release profile
- Easily applied as a coating on drug-eluting devices
APPLICATIONS
- Drug-eluting balloons and stents
INTELLECTUAL PROPERTY STATUS
- Provisional application (April 2023)
PROJECT STATUS
Proof-of-concept studies have been conducted. The chimeric peptide has been demonstrated to prevent migration of rodent or human smooth muscle cells, without affecting endothelial cell migration, in a scratch-wound assay. No demonstrated toxicity was observed in cell viability assays with smooth muscle or endothelial cells. The mechanism of action has been characterized. Peptide treatment reduced intimal thickening in balloon-injured rat carotid arteries at 1 or 2 weeks after injury. Re-endothelialization of the injured vessels was not impaired. A nanoparticle delivery system has been developed for slow release of the chimeric peptide. Early, in-vivo preclinical studies are consistent with preliminary in-vitro observations.